The use of containment isolators in drug manufacturing has increased significantly in recent years. And the pharmaceutical isolator market is expected to grow by 11 percent annually up to 2030, reaching $4.17 billion. Much of this demand is driven by an increased use of potent ingredients in oncology treatments; indeed, relatively recent developments in targeted cancer therapies have led to an expansion of precision medicine production, which often relies on highly potent active pharmaceutical ingredients (HPAPIs).
Since HPAPIs are hazardous materials, they must be treated with care to ensure that i) operators remain protected while handling, and ii) cross-contamination is avoided. As always, regulatory authorities also want to see that manufacturing processes meet appropriate quality and safety standards.
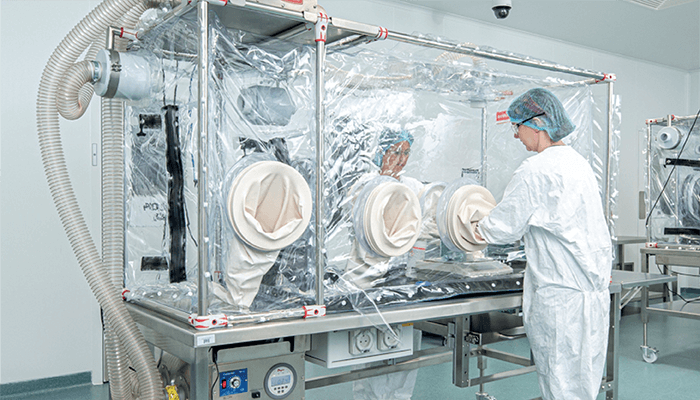
One rapid way to incorporate isolation technology into existing production lines is through deployment of flexible containment solutions. Flexible containment solutions are those that are designed for single use and made bespoke to the needs of the manufacturer, while still ensuring the protection of both the user and the product in a contained environment. The initial growth in uptake of flexible containment technology centered around its ability to contain existing production lines. Such technology can be retrofitted to individual processes to separate operators from ingredients and have been independently tested and proven to achieve less than 1 µg/m3 (OEB 5) and support products with OELs in the nanogram range.
As manufacturers conduct their own tests and equipment containment standards, businesses now see the technology as a cost effective option. The manufacture of highly potent compounds requires intensive cleaning to remove any trace of the previous product before a new product is manufactured. Such cleaning processes are costly and time-consuming. But flexible containment solutions use single-use elements, such as glove bags, or allow strip down and cleaning of mills and compactors while still in the isolator, which reduces the risk of cross contamination, drives down validation costs, and reduce downtime of the production lines.
What’s more, flexible containment solutions are continuously evolving – in terms of aesthetics and ergonomics through improved films materials, but also in terms of advanced control functions. Modern systems can now control humidity, temperature, oxygen degradation, and UV light through the integration of automated and non-automated control systems. These advances allow flexible containment solutions to support a range of drugs. For example, dry powder inhalation formulations (DPIs; a dosage form containing micronized drug particles small enough to be deposited in the lungs) need controlled/minimized exposure to moisture during the production process of these drugs because excess moisture can have a dramatic effect on the delivered therapeutic dose to the patient.
In my view, flexible containment solutions have evolved enough to be considered a first-choice option for specific use cases. As new manufacturing facilities are built with HPAPI drug production in mind – a field that is only expected to grow, I believe flexible solutions will play a key role in ensuring that life-saving oncology drugs can be produced safely and efficiently.