Pharmaceutical operations are optimized to produce high quality drugs reliably over a period of decades. Introducing innovations into manufacturing can lead to long-term risks, so any necessary changes demand thorough consideration. In particular, medical safety and economic viability have to be analyzed in parallel when choosing the primary packaging and the corresponding fill and finish concept. Assessing total cost of ownership is a good approach to gauge the economic viability of a project that spans several years, particularly when choosing between ready-to-use (RTU) and traditional containers. SCHOTT has developed a straightforward model to help customers select the ideal filling setup for their operation – based on an analysis of the total costs of ownership.
The pharmaceutical industry is at a pivot point. Of the more than 4500 injectable drugs in the pipeline, approximately 80 percent are biologics (1). These are mainly targeted towards smaller patient populations, addressing rare diseases or enabling a more patient-centric therapy. At the same time, the more rapid market entry of biosimilars increases the importance of drug life cycle management. Batch sizes are getting smaller, changeovers on fill-and-finish lines are becoming more frequent, and adaptations in primary packaging container formats are more likely. These trends require high flexibility, which can be provided by pre-washed, pre-sterilized standardized RTU containers. However, there are cases where a switch from traditional to RTU containers is not economically feasible. To account for this added complexity, we must gain a thorough and holistic understanding of all options and parameters.
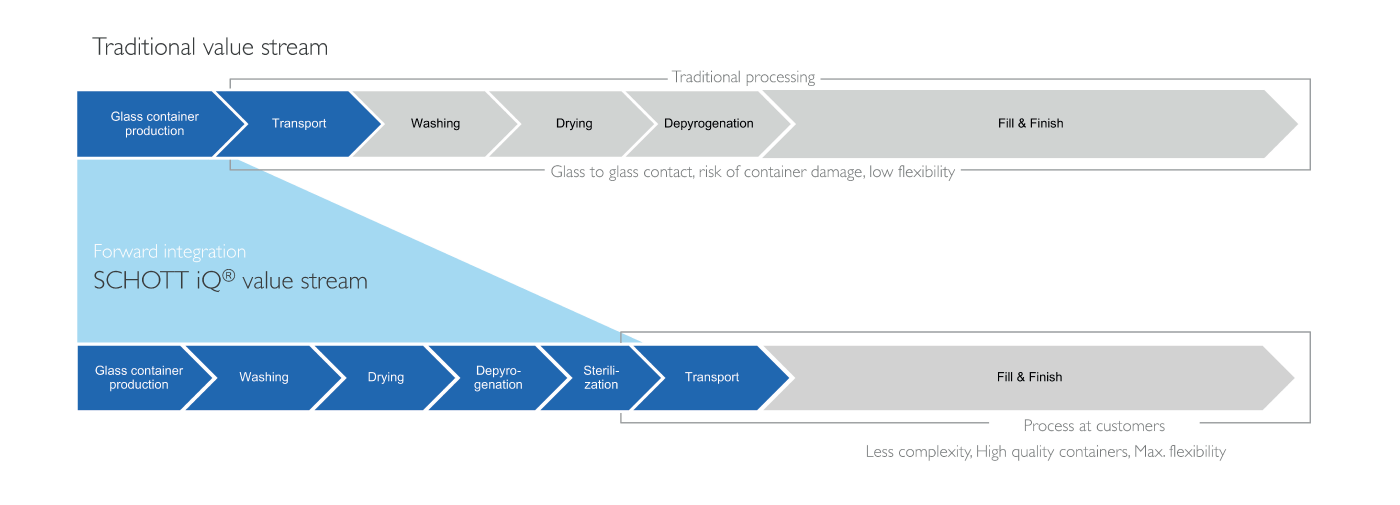
Unlike traditional solutions, RTU containers come washed, depyrogenated, and sterilized, which means they can go straight into the filling operation, eliminating many steps for pharma companies (see Figure 1). The containers are delivered in a nest-and-tub configuration, which also prevents glass-to-glass contact, and reduces defects, such as scratches, breakage, and particles. The nest-and-tub concept was first developed for RTU prefilled syringes and has been the industry standard for around 30 years. Today, SCHOTT offers the whole range of primary packaging containers in its SCHOTT iQ® platform within a harmonized and standardized secondary packaging. Pursuing a single, standard method of filling allows manufacturers to maximize the utilization of each filling line (2). And that leads to greater flexibility, which is further enhanced through increasingly flexible machine concepts.
The traditional value stream for filling pharmaceutical containers is relatively straightforward. Glass containers are produced and transported to the pharmaceutical manufacturer, where they are fed into the filling line, washed, dried, and depyrogenated to ensure the containers are safe and sterile, ready for the fill-and-finish process. As all container types have different dimensions, separate filling lines are required in individual cleanrooms for vials, prefilled syringes (PFS), and cartridges. This translates into higher initial investments, greater operating costs, and reduced flexibility. In addition, the glass-to-glass contact throughout the process carries a risk of defects, breakage, and particle generation.
With RTU containers, the value stream is forward integrated to reduce complexity and maximize flexibility for the pharmaceutical company, as all the containers fed into the filling line have already gone through a standardized, validated and cost-optimized washing and sterilization procedure. Because the SCHOTT iQ® platform is built on compliance with all relevant industry standards, the different container formats can be filled on a single filling line, which significantly reduces the initial investment in machinery and cleanroom capacity.
Economic viability is best assessed by looking at the total cost of ownership, which includes all initial and recurring costs throughout the whole project lifetime. For fill-and-finish operations in the pharmaceutical industry, SCHOTT developed a straightforward model and calculated the total cost of ownership for different scenarios using RTU and traditional containers. The model allows us to guide customers to find the right packaging configuration for their business case.
SCHOTT’s model is based on the typical costs that occur during a pharmaceutical fill-and-finish operation, as listed in Table 1. All capital expenditures (CAPEX) are taken into account, with a depreciation rate appropriate to the project lifetime, such as the cost of the filling line and the washer, and setting up the cleanroom space. The recurring operational expenses are also included, such as primary packaging or labor costs. By comparing typical relative values between traditional and RTU filling, the impact of the individual cost positions becomes apparent. Though filling traditional containers is associated with lower expenses for primary packaging, RTU filling offers advantages of no direct costs for washing and sterilization, as well as lower costs associated with the risk of glass breakage and cosmetic rejects caused by glass-to-glass contact. Similarly, fewer personnel are needed to operate the higher automated RTU filling lines.
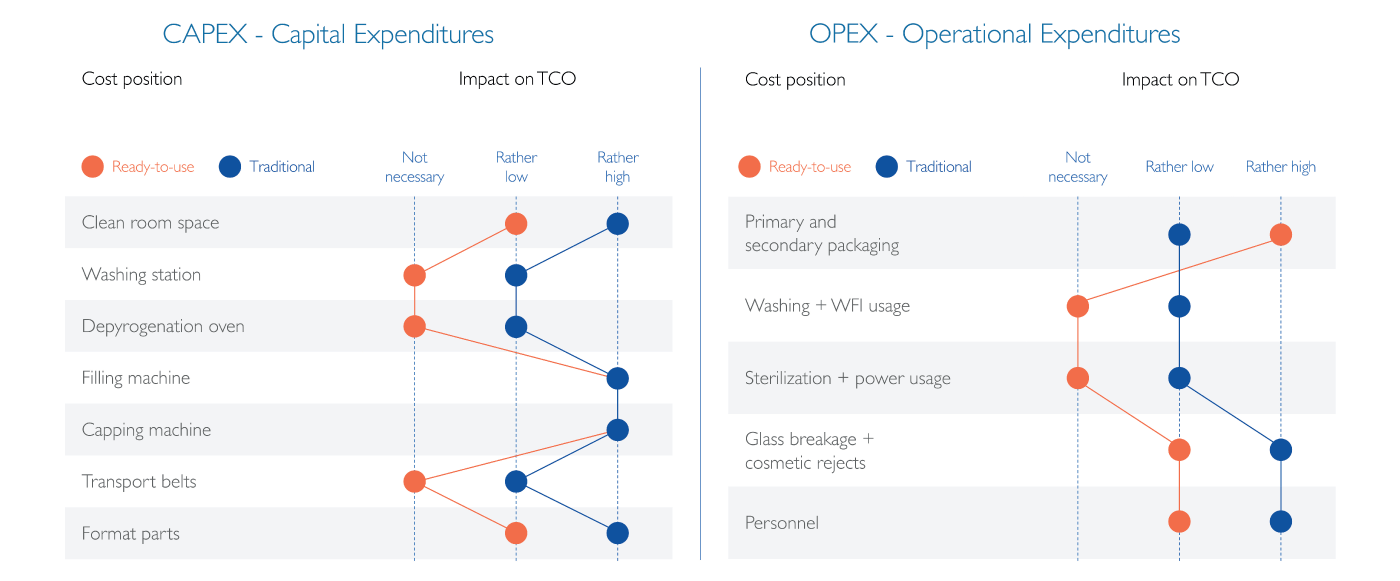
When trying to decide which filling strategy to follow, all costs have to be carefully balanced against each other. In SCHOTT’s TCO model, the above-mentioned costs are determined based on several simple questions:
- Where will the factory be?
- How long is the project duration?
- Which and how many containers of different types will be filled?
- Which machines will be used?
- How many shifts will be in operation?
- Will it be a small batch or campaign production?
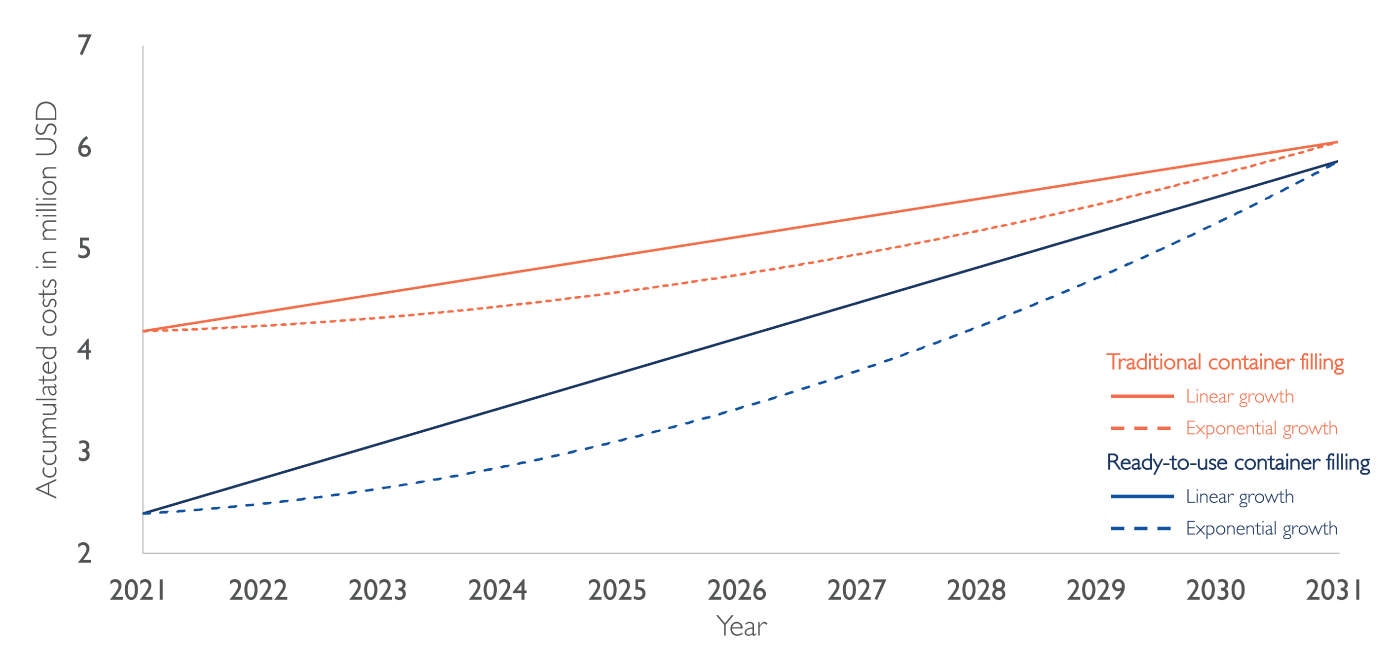
With these inputs and database values for the different cost positions, the total cost of ownership can be modeled for different growth scenarios. Figure 2 shows an exemplary case calculated using this model. The project, starting in 2021 with an initial investment of around US$2 million, is modeled with a linear growth (solid line) and an exponential growth (dashed line). It can be seen that for both growth scenarios, filling only RTU components is cheaper at the end of the 10-year project timeframe. The economic advantage of filling RTU containers can be partly attributed to the higher costs associated with the need to buy multiple filling lines for the different container types and greater cleanroom space requirement.
Deciding on a fill and finish concept that needs to be economically beneficial for several years or even decades is becoming more challenging, with new container options entering the market and the increased need for flexibility. Looking at the total cost of ownership helps obtain a clear picture of the machine and container combination that facilitates operation throughout the lifetime of the project. And SCHOTT’s straightforward TCO model – based on extensive knowhow of an industry leader – helps you come to a sound conclusion.
References
GlobalData, 12/2020 European Pharmaceutical Manufacturer, “Money talks,” (2014). Available at www.epmmagazine.com/money-talks