When it comes to viral safety, biopharma companies have historically focused on their downstream processes, but recently there is increased awareness of the potential disruption viral contamination can bring, and a move towards mitigating the risk in upstream cell culture processes. The key driver for all aspects of viral safety is, of course, patient safety, and there are few regulatory pressures pushing companies to incorporate viral risk mitigation technologies upstream. Regulatory guidance focuses on ensuring cell lines and raw materials are well-characterized and free from detectable adventitious agents. However, there is a good business case to implement measures that minimize the risk of viral contamination and the potential disruption to manufacturing operations.
Where can contaminants enter the upstream process? Chinese hamster ovary (CHO) cells are especially susceptible to contamination with rodent viruses; minute virus of mice (MVM), in particular, has led to problems at a number of biopharma plants. Contamination often originates from raw materials and animal-derived components such as bovine serum or trypsin, which are regarded as particularly high-risk. Contamination can also originate from other cell culture components such as glucose – which attract rodents – or from equipment or facility operators, who can introduce human virus contaminants (such as adenovirus) into a process (Figure 1, viral risk identification).
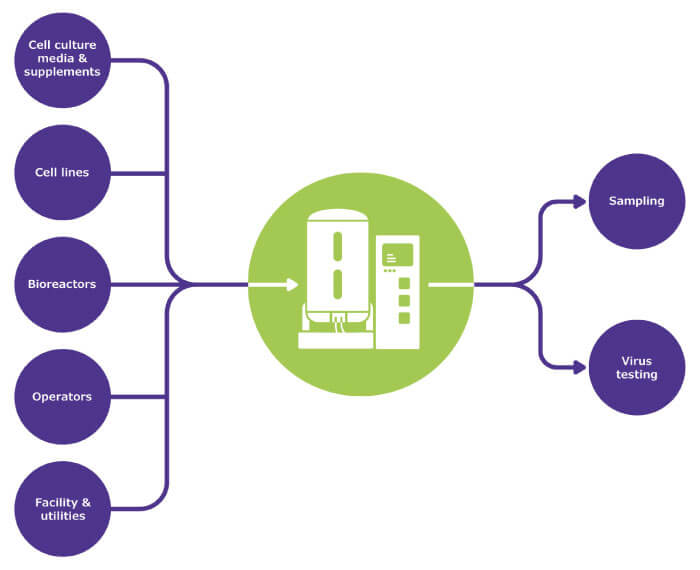
The multitude of potential contamination sources necessitates a risk mitigation strategy built on complementary elements that prevent contamination and include both the raw materials and the manufacturing environment (Figure 2, viral risk mitigation strategies upstream).
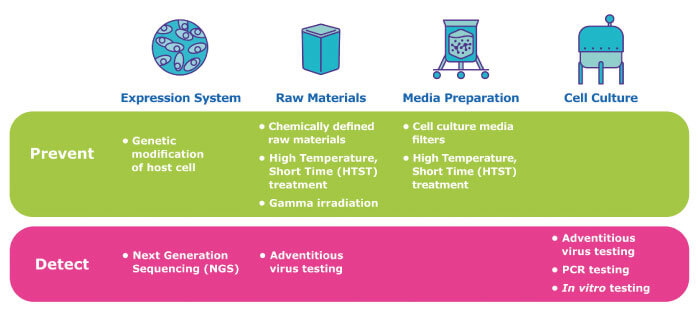
A recently introduced option to reduce contamination risk is the availability of genetically modified CHO parental cell lines that have been engineered to eliminate the receptors used by the virus to enter cells, rendering them resistant to MVM infection. More traditional approaches for raw materials focus on sourcing, selection and treatment. Wherever possible, animal-derived cell culture components at high risk of virus contamination should be replaced with lower-risk alternatives, such as non-animal origin supplements, or recombinant proteins where the production processes reduce concerns about adventitious agent contamination. Where animal-derived components must be used, they should be carefully sourced from lower risk geographies. However, no material, even plant-derived or of recombinant origin, should be considered risk-free, as contamination may occur at any time throughout the supply chain of the raw material.
An additional virus risk mitigation option is to treat raw materials to inactivate potential viral contaminants before they enter the manufacturing facility - a “point-of-origin” strategy. For instance, bovine serum can be treated with gamma irradiation to inactivate viruses, and glucose can be treated with high-temperature short time (HTST) pasteurization to inactivate viruses – even those with high physico-chemical resistance. The recent availability of HTST-treated glucose manufactured under an ISO9001:2015 comprehensive quality management system is a new option for biomanufacturers, which may eliminate the need for significant capital investment and establishment of HTST technologies in-house.
In addition to “point-of-origin” approaches, the risk of viral contamination of cell culture processes can be reduced by implementing “point-of use” strategies that focus on treating materials immediately before they are used in the bioreactor. Filtration and HTST treatments of cell culture media are examples of point-of-use strategies that remove or inactivate potential adventitious organisms from cell culture media preparation. The recent development of virus-retentive filters specifically designed to process cell culture media offer additional benefits to traditional sterilizing-grade filters for reducing the risk of introducing viral contaminants into the bioreactor. These filters efficiently process many different cell culture media, while delivering high retention of viruses and mycoplasma and sterilizing-grade performance for bacteria, all with low capital investment.
Perhaps the most effective approach to prevent contamination is to work with suppliers who follow high quality standards with transparency and visibility of their supply chain. Understanding the origin of raw materials and how they are controlled in a quality management system provides peace of mind.
Although there are several good options to reduce the likelihood of virus contamination, risk cannot be entirely eliminated. All comprehensive virus risk reduction strategies depend on a sensitive panel of virus assays capable of detecting contamination if it is present. Regulatory guidance documents provide a detailed framework of testing expectations for biologic products.
Traditional cell-based assays remain the standard approach to biosafety and viral testing, but biopharma manufacturers are increasingly being drawn to newer molecular methods, such as broad specificity polymerase chain reaction (PCR) and next generation sequencing (NGS), to expedite the viral safety testing process. These newer testing strategies can provide greater confidence in viral testing results and opportunities to accelerate testing.
Bioprocesses are also evolving – and connected, continuous, intensified, and more automated processes present some particular challenges to minimizing contamination risks of viral contamination. For example, the high media requirements for intensified processes increases the possibility of viral contamination. In addition, because steps are linked together, problems can ripple through the process – potentially to downstream operations. These challenges are best met with technologies that enable rapid, real-time monitoring of the upstream process.
Finally, it is also worth drawing attention to the advantages of implementing single-use technologies in upstream processes. Single-use technologies such as bioreactors, connectors and sampling devices are pre-sterilized with gamma irradiation before use, reducing the risk of introducing contaminants into the operation. In addition, if contamination occurs during processing, contaminated material and components can be rapidly disposed of. Single-use technologies eliminate much of the cleaning that might be required in the event of contamination in more traditional stainless-steel systems, enabling manufacturers to get back on-line faster. In an industry where time is money, single-use systems and components offer both flexibility and other advantages to manufacturers.
Traditionally, preventing upstream viral contamination has focused on sourcing and testing raw materials. This approach to risk mitigation has worked well for many years and, to date, no contaminated biopharma products have reached a patient. For manufacturers, however, viral contamination events are incredibly disruptive and expensive; high-profile cases are driving companies to re-examine their risk assessments around virus safety and take steps to reduce risk upstream of the bioreactor.
There is no single viral safety solution that works for every process – solutions depend on the process, media components, facility, scale, and the type of biologic being produced. Multiple strategies and technologies to mitigate risks should be integrated into an overall virus safety management program – based on effective risk analyses. In short, a holistic approach that considers all components of the manufacturer’s process, is the only reasonable approach to minimize pathogen safety risk.
MilliporeSigma is a trademark of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources. The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the US and Canada.





