Experts recently gathered at Merck’s site in Irvine, Scotland, to discuss challenges and innovations in upstream bioprocessing and to share best practices. Although antibody titers have increased markedly, there are still many areas where upstream production processes can be improved. In particular, there is considerable discussion in the industry about how to reduce the cost of therapeutics – and getting upstream development right the first time is one way to achieve this. In addition, other approaches such as intensified or continuous bioprocessing, are attracting significant attention in the industry as new technologies emerge that will make this a reality.
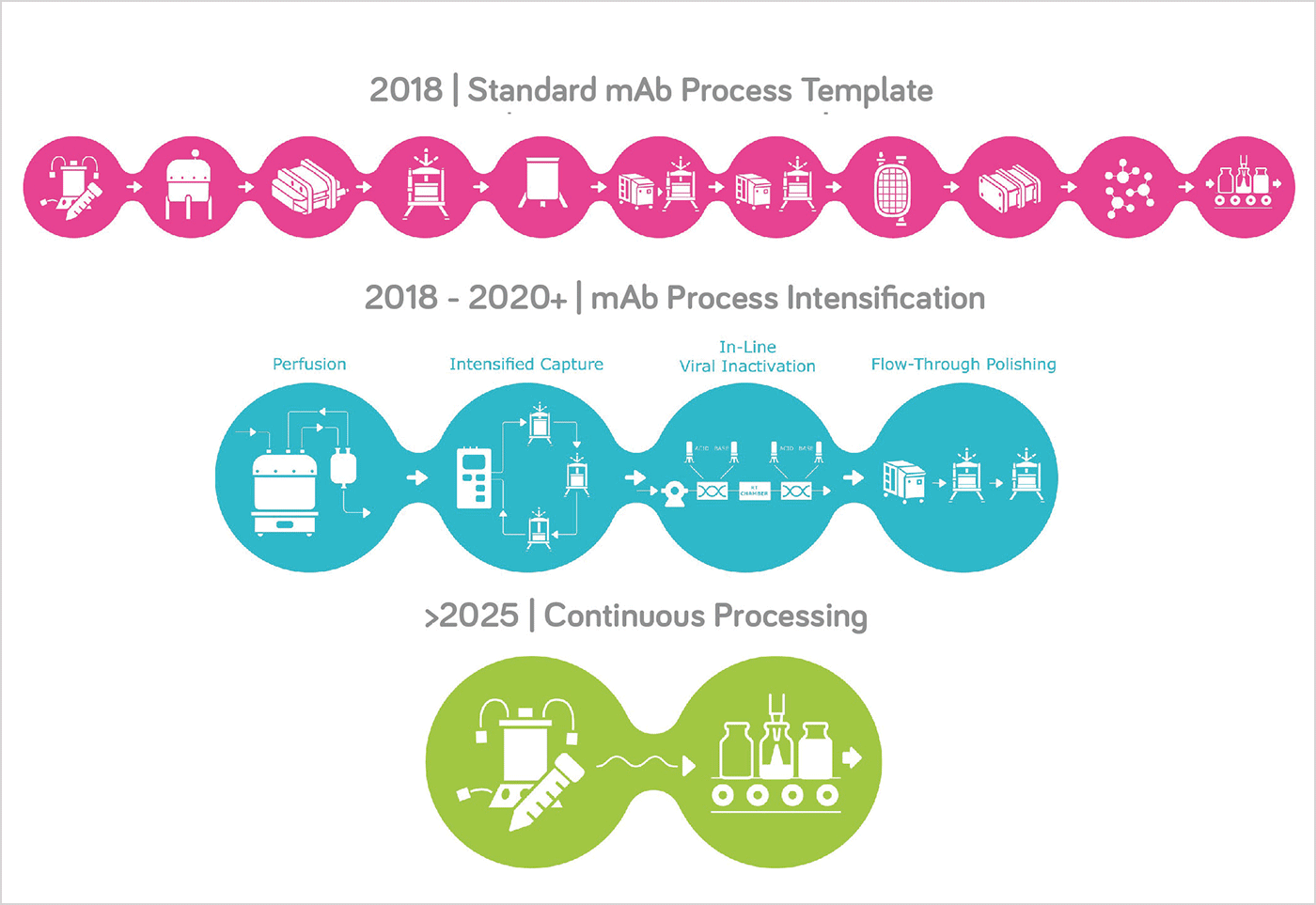
Some of the topics discussed at the Upstream Innovation Day included development of the optimal cell culture media for specific processes, next generation bioprocessing, supply chain interdependencies, and the facilities of the future. Presentations were given by drug manufacturers, consultants, and Merck experts. In my view, intensified, connected and continuous bioprocessing is a key trend for the future. To this end, Merck presented its BioContinuum™ Platform (www.MERCKmillipore.com/biocontinuum), a program that incorporates connected software, controls and analytics across our portfolioof proven and novel technologies empowering biomanufacturers to achieve greater speed, flexibility and reliability through intensified, connected or continuous bioprocessing. This program enables them to confidently enter the era of industry 4.0 and to make the factory of the future a reality.
Contaminations costs
The Upstream Innovation Day coincided with the site’s recent expansion to utilizing high-temperature, short time (HTST) technology for the treatment of glucose for virus inactivation. The concept of sterilization using heat is well known, but in biopharma it is very difficult to implement without denaturizing protein products. With our validated HTST capability, we can accommodate from small batches of just a few liters up to commercial scale of 10,000 L.
New options for preventing viral contamination was another topic at the event. Of all the challenges upstream, preventing viral contaminations remains a significant concern because, although rare, contaminated bioreactors can be devastating. A low level of contamination is enough to bring down a bioreactor, wasting highly valuable product and a lot of time in investigations, decontamination, and corrective actions. Although many biopharma manufacturers are moving to chemically-defined media and removing serum or other animal-derived components from their processes, viral contaminations still occur.
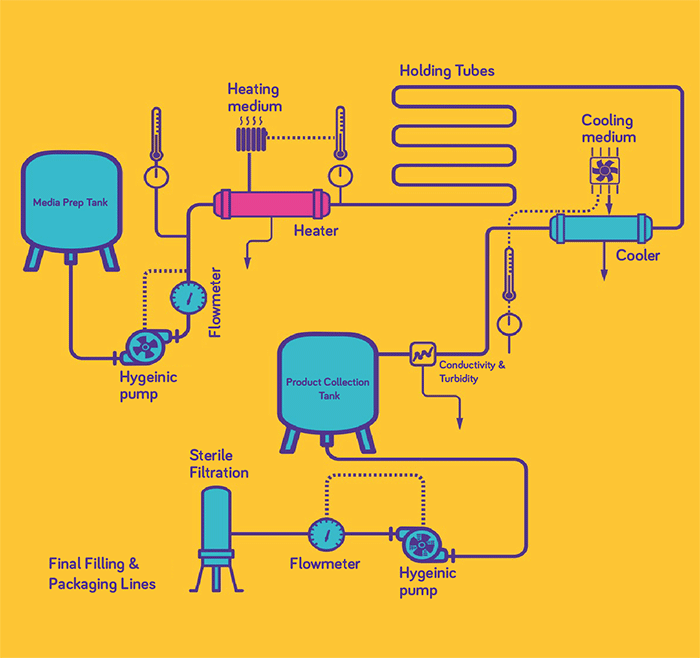
Glucose and other sugars, which are present in high concentrations in cell culture media, are often considered highrisk components. These components are rodent attractants and are at risk of contamination throughout the supply chain. A well-controlled supply chain is essential to reducing the risk, but biomanufacturers also look to other risk control solutions. HTST technology can inactivate viruses in glucose solutions without impacting reagent performance, offering a new option for improved viral safety in upstream processes. Importantly, HTST-treated glucose is virus free when it enters the biomanufacturing site.
Following the trend for improvement
Merck is in a unique position in the industry with a longstanding history of designing and developing innovative new products and technologies to meet our diverse customer needs. Our extensive network of technical experts provides end-to-end services to biopharmaceutical manufacturers on a variety of projects from pre-clinical through to pre-commercial across a range of different applications. Deep customer relationships and global reach enable a wide perspective on the issues that matter to biopharma manufacturers. From continuous bioprocessing, to viral contaminations solutions, to controlling trace metals in formulations, we are keeping our eyes on the industry from all angles.
Omar Wahab, PhD, is Head of Franchise. Upstream Cell Culture & Biopharm Materials at Merck.
Merck, and BioContinuum are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.





