Monoclonal antibodies and other biopharmaceutical products, as well as their manufacturing processes, are inherently at risk of viral contamination, making viral safety testing critical. Viral safety testing is mandated by regulators worldwide, and although technologies for biomanufacturing have rapidly advanced, viral testing methods remain largely the same today as they were thirty years ago. Traditional virus detection approaches – cell-based assays – have served the biopharma industry very well over the years, but they have limitations; for example, some assays have long turn around times such as 28 days. In addition, although cell-based assays can detect contaminants, they generally cannot directly identify them and it can be slow to obtain results.
Albert Einstein once said, “Once we accept our limits, we go beyond them.” In an age where speed is the key to success, we believe it is time to accept the limitations of traditional testing and to focus on newer technologies that focus on speed, sensitivity and reliability. Faster assay results will lead to more rapid batch disposition, reduced interruption of processing, and also meet the needs of more intensified processing – a key capability given the increasing interest that manufacturers are paying to continuous manufacturing strategies.
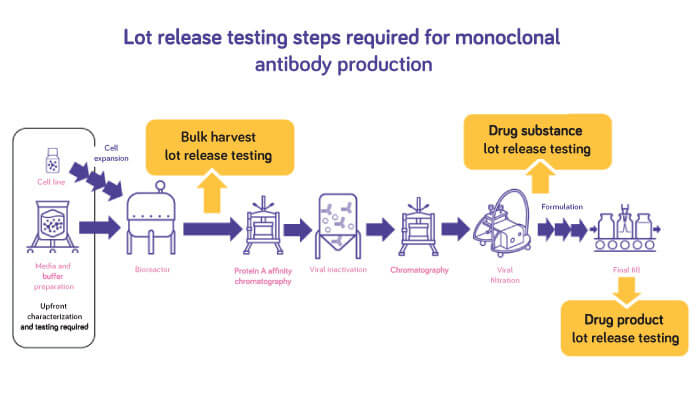
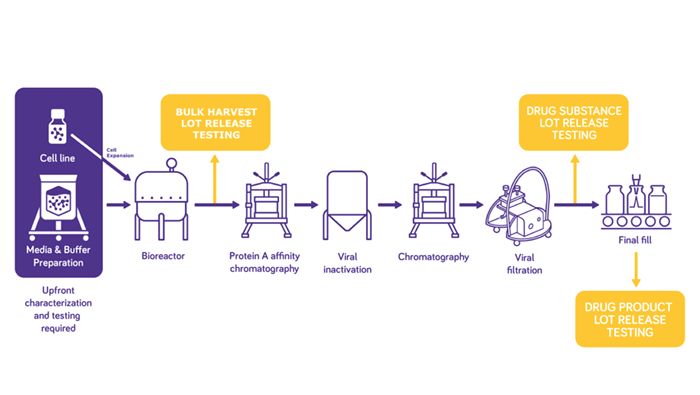
Although traditional assays remain the standard approach to biosafety and virus testing, biopharma manufacturers are increasingly being drawn to molecular methods such as broad specificity polymerase chain reaction (PCR) and next-generation sequencing (NGS), to expedite viral safety testing.
Of all the molecular methods available, we think it’s fair to say that NGS is the one that excites the industry. Many biopharma organizations employ NGS extensively in early stages of development for cell line characterization. Although the technology has been available for well over a decade, its use in biosafety testing is much more recent – and has only become feasible as sequencing costs have lowered and implementation methods have become standardized.
NGS is so effective as a molecular tool because it enables de novo identification of both known and unknown agents (viral, bacterial, or fungal) with precision and sensitivity. MilliporeSigma was the first to provide a GMP compliant NGS offering paired with a fully validated bioinformatics platform. However, despite these advantages, currently NGS tends to only be used where traditional testing approaches struggle or fail – for example where a product may be incompatible with cell-based viral detection approaches. However, for newer virus-based therapeutic products, where traditional assays are more challenging, NGS is an attractive alternative to meet virus testing requirements.
PCR enables detection of DNA or RNA sequences in vitro. It has been used in biosafety testing for the past twenty years, with the biggest advantages being that it is rapid (results available in a few hours) and highly sensitive. The largest issue with traditional PCR, however, is that small changes in the sequence of the target organism genome may result in a failure to amplify and potentially, a false negative result. The application of PCR in biosafety testing has evolved, with quantitative real-time PCR and, more recently, digital PCR approaches, allowing for more sensitive detection and more accurate quantitation of nucleic acid levels.
Other approaches are also enhancing the potential of PCR methods for virus testing. At MilliporeSigma, for example, we are working on broadening the detection capability of PCR by developing degenerate primer sets that can broadly detect the seven families of DNA viruses and 14 families of RNA viruses relevant to CHO manufacturing. This novel approach using familiar technology enables us to identify a contaminant in a single test, rather than having to perform multiple different PCR tests. In our view, this expands the breath of detection while keeping the sensitivity and speed of PCR, opening up a huge opportunity to accelerate biosafety testing.
As both NGS and PCR methods evolve, they present clear opportunities to accelerate virus testing, which is meeting the needs of an industry that is looking for real-time decisions and information on the quality of the drug being manufactured. Indeed, these methods and other rapid testing technologies, such as biomonitoring and pyrogen detection, enable biopharmaceutical manufacturers to control their most important commodity – time.
A central conflict that we often see is that, although willing, biopharma manufacturers are often hesitant to implement new testing technologies due to concerns over regulatory implications. However, the regulatory documents on biosafety testing encourage the implementation of methods where it is demonstrated that the method is as good as, or better than, an existing technology; and that it meets the intended purpose of testing. The good news is that with the rise of cell and gene therapies, regulators are more frequently exposed to alternative and rapid testing strategies as traditional approaches are often not compatible with these modalities and the newer methods offer the only viable option for viral safety testing.
As any manufacturer will tell you, development of testing methods is only half the story. At MilliporeSigma, we are investing on the development of new biosafety methods and we also validate the performance of these tests to ensure they meet stringent GMP standards, thus bringing the confidence drug manufacturers need to use them.
Testing methods and approaches will continue to advance but the next revolution in viral safety testing may come sooner than we think. As biomanufacturing is moving to connected, continuous, intensified, and more automated processes, the notion that these highly developed manufacturing processes can wait for the time-to-results from traditional adventitious virus assays seems unlikely. The processes of tomorrow are looking for testing that can provide real-time test results enabling fast lot release, without compromising quality.
A current buzz in the industry is in-line testing, where testing is performed within the bioreactor environment for both ongoing monitoring as well as bulk harvest lot release. Realistically, not all technologies can be implemented this way and, to meet the needs of rapid time to results, some tests must evolve from being run in a testing lab away from the manufacturing site, to being able to be run close to the manufacturing line. We call this near-line testing. As the technologies develop, they can be brought ever closer to the manufacturing process, with testing on the manufacturing floor, or at-line. Our current thinking is that the closest these test technologies can get will be on-line, where a sample is taken from the process and consumed within a fully automated test. It is only with this evolutionary approach that virus testing timelines can reduce from days to hours, thus enabling intensified and ultimately continuous manufacturing processes (Figure 2).
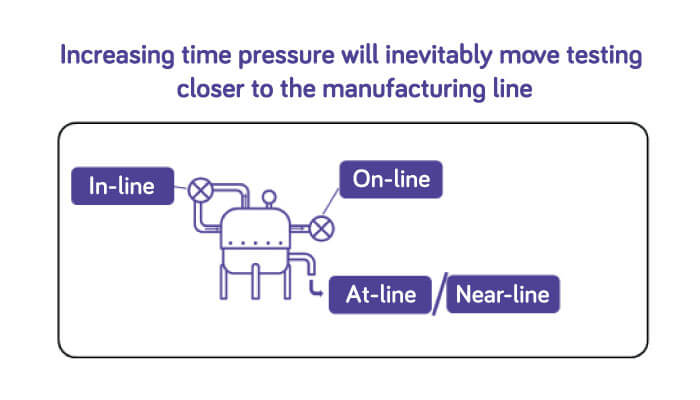
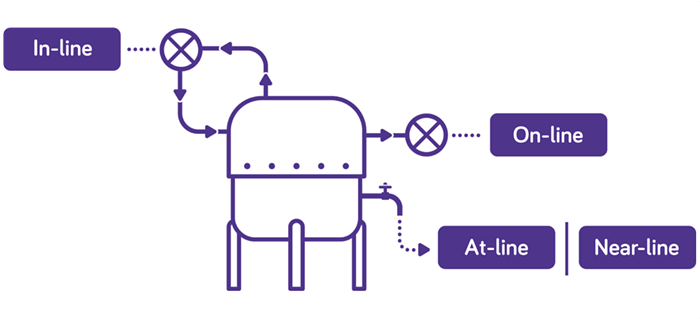
Our teams of technical experts have been proudly supporting the biopharma industry for over 70 years with BioReliance® biosafety services and are committed to developing tests and services to support the evolving biologics market. Our experts understand the different needs of the processes that comprise drug product manufacturing and will work with you to design a solution that fits your needs.
All authors focus on the BioReliance® biosafety portfolio at MilliporeSigma. The life science business of Merck KGaA, Darmstadt, Germany operates as MilliporeSigma in the U.S. and Canada. MilliporeSigma and BioReliance are trademarks of Merck KGaA, Darmstadt, Germany or its affiliates. All other trademarks are the property of their respective owners. Detailed information on trademarks is available via publicly accessible resources.





