Brexit talks are at an impasse over the question of the Irish border. And unless a mutually acceptable “backstop” – to prevent the need for new infrastructure at the Irish border – can be found, there will be no Withdrawal Agreement, no transition, and no deal. As of March 30, 2019, the UK would become a “third country,” where all EU primary and secondary law ceases to apply. For marketing authorization holders (MAHs) in the UK, steps must be taken now to ensure that products can remain on the EU market after March 29. These include: transferring the marketing authorization to a MAH based in the European Economic Area (EEA), as well as changing the location of the Qualified Person for Pharmacovigilance (QPPV), pharmacovigilance system master file (PSMF), batch release, quality control and importation sites, to the EEA.
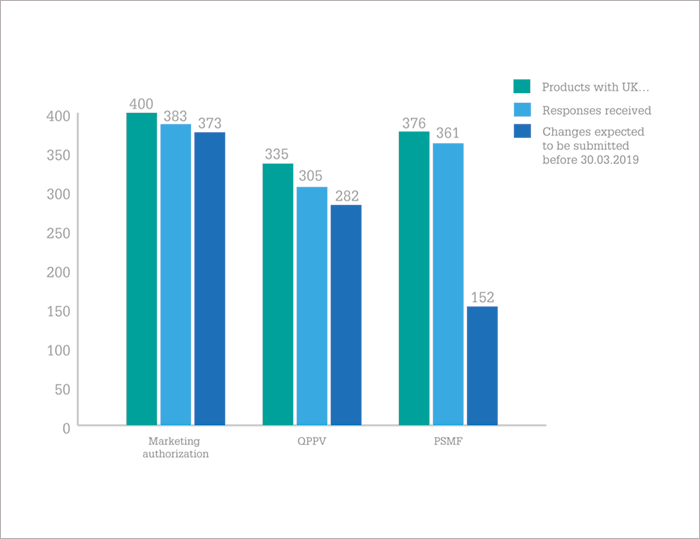
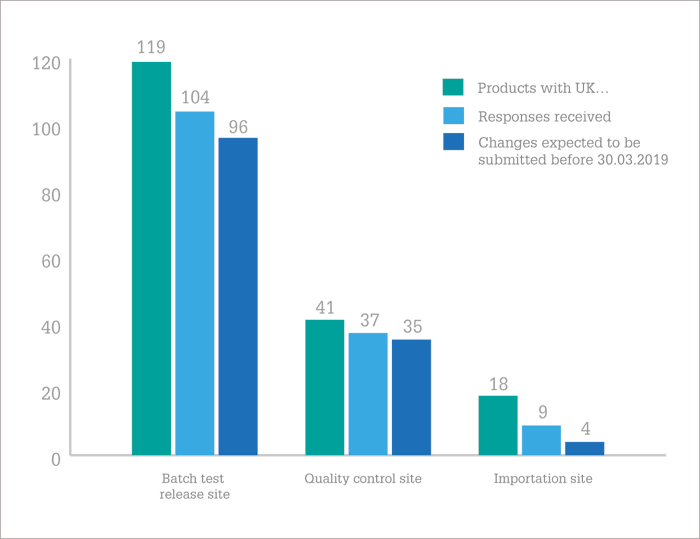
So, are companies on track? In January 2018, the EMA contacted over 180 MAHs of 694 human and veterinary centrally authorized medicinal products to find out. Their results, published in July (1), found that the majority (58 percent) of UK marketing authorization holders (MAHs) are on the ball, but there are “serious concerns” from the EMA that the necessary steps won’t be taken in time for 16 percent of products. The EMA is “strongly advising” pharma companies to submit the necessary changes for the continued maintenance of their marketing authorizations to EMA as soon as possible – at least before the end of Q4 2018. The EMA will now follow-up directly with MAHs that do not plan to submit the changes required before March 30, 2019, to avoid potential supply disruptions.
References
- EMA, “Report from EMA industry survey on Brexit preparedness”, (2018). Available at: http://bit.ly/2vriaQW. Accessed August 1, 2018.




