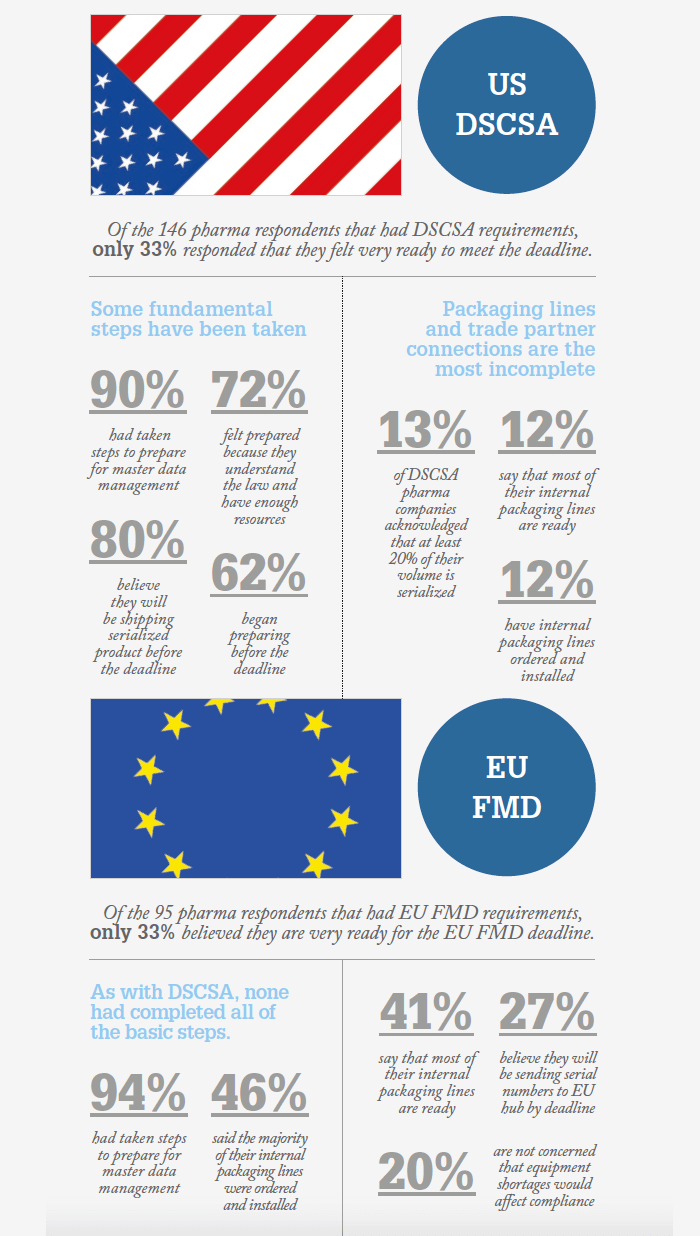
The US and EU serialization deadlines are both less than a year away, with the EU Falsified Medicines (FMD) Directive coming into force on February 9, 2019 and the US Drug Supply Chain Security Act (DSCSA) coming into force on November 27, 2018 – the latter already having been delayed by one year. So how prepared are pharma companies?
Tracelink surveyed 660 companies across segments of the supply chain, including 174 drugmakers, to find out. In their Global Drug Supply, Safety and Traceability Report, they found that only one-third of respondents believe they are “very ready” for serialization. The data also revealed that no single company has actually completed all the basic steps for serialization readiness. According to the authors, the survey proves “that the companies who feel very ready are considerably ‘less ready’ than they think.”
References
- TraceLink, “TraceLink Publishes Full Analysis from the Global Drug Supply, Safety and Traceability Report, Industry’s Largest Survey on Life Science Companies’ Readiness for Serialization Deadlines” (2018). Available at: https://bit.ly/2JvuQfP. Accessed April 10, 2018.




