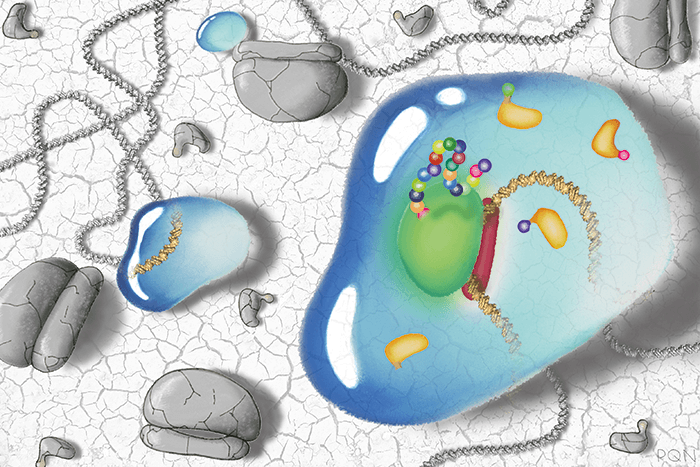
Large-scale centralized manufacturing has helped bring cheaper pharmaceuticals to millions of patients, but for those in remote locations, access remains a problem – especially for medicines that require a cold chain. Increasingly, however, scientists are looking for ways to circumvent the problem by manufacturing drugs at the point-of-care. In 2014, James Collins, a faculty member at the Wyss Institute at Harvard University and the Henri Termeer professor of medical engineering and science at Massachusetts Institute of Technology, and his colleagues developed a method for producing therapeutic molecules on-demand with freeze-dried synthetic gene networks (1) – the reaction is kick-started simply by adding water. Earlier this year, they used the technology to develop diagnostics that could detect Zika viral RNA at clinically relevant concentrations (2), and now, in their most recent study, they have used the technology to produce complex biomedicines (3). We asked Collins to tell us more.
References
- K Pardee et al. “Paper-Based Synthetic Gene Networks”, Cell, 4, 940-954 (2014). K Pardee et al. “Rapid, low-cost detection of Zika virus using programmable biomolecular components”, Cell, 165, 1255-1266 (2016). K Pardee et al. “Portable, on-demand biomolecular manufacturing”, Cell, 167, 248-259 (2016).




