INTRODUCTION
During the last two decades, biomedical research at a worldwide level has been drastically increasing its efforts to develop nanoparticle based drugs and bring them from the bench to the bedside. Today, many high-potent molecule-based drugs are used in suboptimal conditions: low dosage (because of their toxicity) or lack of efficiency in the targeting specific organs, cell types, etc. Thanks to recent discoveries, it has been recognized that nanoparticles, like polymers or liposomes, have excellent properties as drug delivery vehicles to address some of these issues. When considering a nanoparticle-based drug it is critical to characterize the size of these vectors, as it has a considerable effect on their pharmacokinetics or their efficiency and ability to reach their target within the body. Equally, it is also imperative to have an idea of the concentration of nano-objects and more importantly the dosage of nano-objects loaded with the drug of interest. Here we describe the use of Nanoparticle Tracking Analysis (NTA) for size and concentration measurements of drug delivery nanoparticles. In addition, by using a fluorescently tagged drug molecule, it was possible to determine how many drug delivery nanoparticles had successfully been loaded with drug molecules.Nanoparticle Tracking Analysis (NTA) Overview
NTA utilizes the properties of both light scattering and Brownian motion in order to obtain the particle size distribution of samples in liquid suspension. A laser beam is passed through the sample chamber, and the particles in suspension in the path of this beam scatter light in such a manner that they can easily be visualized via a 20x magnification microscope onto which is mounted a camera. The camera, which operates at approximately 30 frames per second (fps), captures a video file of the particles moving under Brownian motion within the field of view of approximately 100 μm x 80 μm x 10 μm (Figure A).
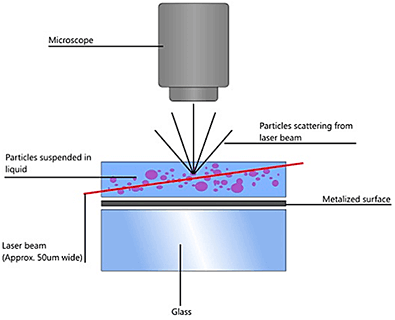
The movement of the particles is captured on a frame-by-frame basis. The proprietary NTA software simultaneously identifies and tracks the center of each of the observed particles, and determines the average distance moved by each particle in the x and y planes. This value allows the particle diffusion coefficient (Dt) to be determined from which, if the sample temperature T and solvent viscosity η are known, the sphere-equivalent hydrodynamic diameter, d, of the particles can be identified using the Stokes-Einstein equation (Equation 1).
NTA is not an ensemble technique interrogating a very large number of particles, but rather each particle is sized individually, irrespective of the others. An example of the size distribution profile generated by NTA is shown in Figure B.
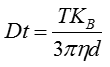
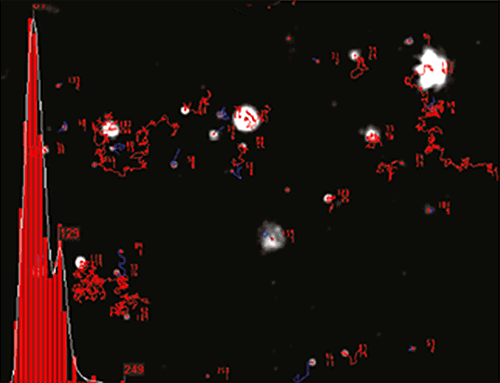
In addition, the particles’ movement is measured within a fixed field of view (approximately 100 μm by 80 μm) illuminated by a beam approximately 10 μm in depth. These figures allow a scattering volume of the sample to be estimated; by measuring concentration of the particles within this field of view and extrapolating to a larger volume it is possible to achieve a concentration estimation in terms of particles per mL for any given size class or an overall total. Finally, a fluorescence mode allows differentiation of suitably labelled particles.
MATERIAL AND METHODS
Equipment:
A NanoSight LM10 HSBF system was used, comprised of a sCMOS camera, 405 nm laser and 430 nm long-pass filter. Analysis was performed using NTA software 2.3.5.
Materials:
Polylactic Acid (PLA) particles (Adjuvatis) were designed, synthesized and loaded with a drug of interest conjugated to a fluorescent label (Coumarin6, Excitation 370 nm; Emission 470 mm) (PLA-Coum).
Method:
PLA-coum nanoparticles were diluted 100-fold with particle-free ultra-pure water* and mixed by pipetting. * The water was checked by NTA before use and found to be free of contaminant particles.
The diluted sample was loaded into the laser sample chamber using a 1 mL silicon-oil free syringe.
The sample chamber was loaded onto the instrument. The image was adjusted to bring the particles into focus and a video was captured using camera level 7 for 60 seconds to generate data for light-scatter (standard) mode (all particles in the sample measured).With the sample chamber remaining on the instrument, the camera level was increased to maximum (level 16), a second 60 seconds video was taken after insertion of the 430 nm long-pass filter and refocussing to obtain fluorescence-mode data (only particles emitting a fluorescence signal measured).
The videos were then processed using a detection threshold of 4 for both scatter mode and for fluorescent mode. The Min Track Length was set up at 6 for both scatter and fluorescence mode.
Size distribution data were obtained for both measurement modes and overlaid for comparison.
>> For results and discussion, follow the link and download of the full Application Note for FREE
SUMMARY
NanoSight NTA measurements enabled a fast evaluation of the size and concentration of polylactic acid nanoparticles. When loaded with fluorescently labelled drug molecules, the subpopulation of particles containing the fluorophore could be distinguished from the total population giving valuable information on the efficiency of the loading process. These data can be used to identify improved loading processes in a quick and easy manner saving time and effort for drug delivery researchers.- NanoSight provides an attractive alternative to more complex methods such Electron Microscopy
- Real time capture provides high resolution size distributions and concentration measurements, revealing both qualitative and quantitative information with the added benefit of direct visual confirmation
- Fluorescence detection mode brings additional functionality allowing data on suitably labelled subpopulations of nanoparticles to be measured
- Minimal sample preparation required
Malvern Instruments provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations and our commitment to maximizing the potential of established techniques. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. Headquartered in Malvern, UK, Malvern Instruments has subsidiary organizations in all major European markets, North America, Mexico, China, Japan and Korea, a joint venture in India, a global distributor network and applications laboratories around the world. www.malvern.com severine.michel@malvern.com





