Introduction
The use of nanoparticles in drug delivery continues to grow rapidly. Nanoparticles offer excellent pharmacokinetic properties, controlled and sustained release, and targeting of specific cells, tissues or organs. Interest in nanoparticle drug delivery is also driven by the diminishing rate of discovery of new biologically active compounds that can be exploited therapeutically to treat disease. With fewer new drugs entering the market every year, interest in the use of nanoparticles’ versatile and multifunctional structures for the delivery of drugs is swiftly increasing. All these features can improve the efficacy of existing drugs (Malam et al., 2011). Nanoparticles used in drug delivery have been defined as colloidal systems of submicron size that can be constructed from a large variety of materials in a large variety of compositions. Commonly defined nanoparticle vectors include: liposomes, micelles, dendrimers, solid lipid nanoparticles, metallic nanoparticles, semiconductor nanoparticles and polymeric nanoparticles. In their many guises, nanoparticles have been extensively employed to deliver drugs, genes, vaccines and diagnostics into specific cells/tissues. (Ram et al., 2011). When considering a nanomaterial drug delivery system, size of the nanoparticle is a key parameter as it directly influences the processes of delivery, uptake, degradation and clearance from the body. For example, nanoparticles in the range of 30 nm to a few hundred nm in diameter can passively accumulate at the site of tumours due to leaky vasculature, phagocytosis favours particles >500 nm, whilst biliary and renal clearance occurs with particles <30 nm and <8 nm respectively. In addition, the liver has a lower uptake of smaller particles (25 nm and 50 nm) compared to larger particles (200 nm and 300 nm). Accurate measurement of the particles being administered is therefore imperative to many systems and processes.Analysis of drug delivery systems by NTA
Liposomes Liposomes (Figure 1) have been the subject of significant research and development efforts for many years and are currently the most common targeted drug delivery system. Liposomes have been approved as a delivery system for amphotericin B for fungal or protozoal infections, doxorubicin for breast cancer treatment, and for vaccines for hepatitis A and influenza. The use and potential of liposomes in drug delivery continues to grow in importance. The reasons are clear:- Therapeutics delivered via liposomes may be protected from the actions of metabolizing enzymes
- Lipophilic substances may be made soluble by the use of liposomes
- Therapeutics can be targeted to specific areas by attaching specific ligands to the liposome
- Liposomes are readily absorbed by cells
- The rate of release may be controlled by the selection of liposome
- Using liposomes as a delivery vehicle allows potentially lower or less frequent doses to be used, potentially reducing toxicity and side-effects
- Liposomes can carry biological substances such as proteins and DNA
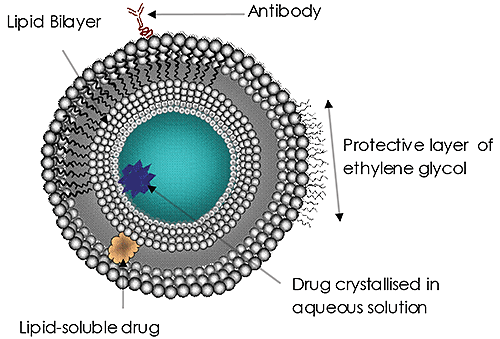
Figure 1. Typical Liposome Structure Sizing liposomes with NTA Malvern’s NanoSight instrument range accurately and rapidly sizes and measures concentration of liposomes in water and other solvents. Only small volumes and very little sample preparation is required. The instruments enable individual liposomes in suspension to be visualized and their Brownian motion tracked – enabling particle size distributions, based on individual particles, to be built up in a matter of seconds. >> Download the full Application Note as PDF
Malvern Instruments provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations and our commitment to maximizing the potential of established techniques. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. Headquartered in Malvern, UK, Malvern Instruments has subsidiary organizations in all major European markets, North America, Mexico, China, Japan and Korea, a joint venture in India, a global distributor network and applications laboratories around the world. www.malvern.com severine.michel@malvern.com





