The application of Quality by Design (QbD) has become second nature to the pharmaceutical industry. The concept of scoping, understanding and controlling a pharmaceutical manufacturing process within the 'design space' is well-established. Alongside this, Process Analytical Technology, most especially on-line measurement, is recognised as playing an important role in delivering the information and understanding to drive QbD, at the pilot scale and through into manufacture. The FDA have encouraged the adoption of QbD by offering, in return, operational freedom within the design space. This enables a responsive approach to understood but unavoidable variability and can substantially enhance manufacturing efficiency. Such gains prompt the question as to whether the principles enshrined in QbD are applicable to other processes, and analytical method development is now a focus. Just like conventional QbD, analytical QbD (AQbD) holds out the prize of flexibility, in contrast to the rigidity of Standard Operating Procedures (SOPs).
The FDA has already released guidance outlining the potential benefits that this flexibility might bring. The view is that the adoption of AQbD will support the development of robust analytical methods which will more easily transfer with the product, through scale-up, from site to site and indeed from instrument to instrument. This represents a considerable incentive for an industry so heavily reliant on rigorous analysis. This whitepaper provides an introduction to AQbD and examines how the process of developing and validating analytical methods can benefit from the systematic and scientific approach that QbD promotes. The development of a laser diffraction particle sizing method is used to illustrate the practicalities.
Introducing the concept of AQbD
The generally accepted definition of QbD, as presented in International Conference of Harmonization document Q8(R2) (ICHQ8), is: 'A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding, based on sound science and quality risk management'. This central idea of a structured and rigorous approach to the development of a process has resonance in the development of analytical methodologies. Figure 1 parallels the QbD and AQbD work flow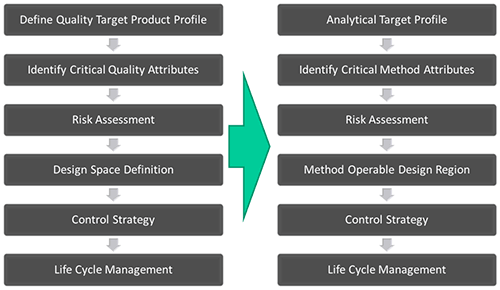
Click here to read the full article
Malvern Instruments provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations and our commitment to maximizing the potential of established techniques. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. Headquartered in Malvern, UK, Malvern Instruments has subsidiary organizations in all major European markets, North America, Mexico, China, Japan and Korea, a joint venture in India, a global distributor network and applications laboratories around the world. www.malvern.com severine.michel@malvern.com





