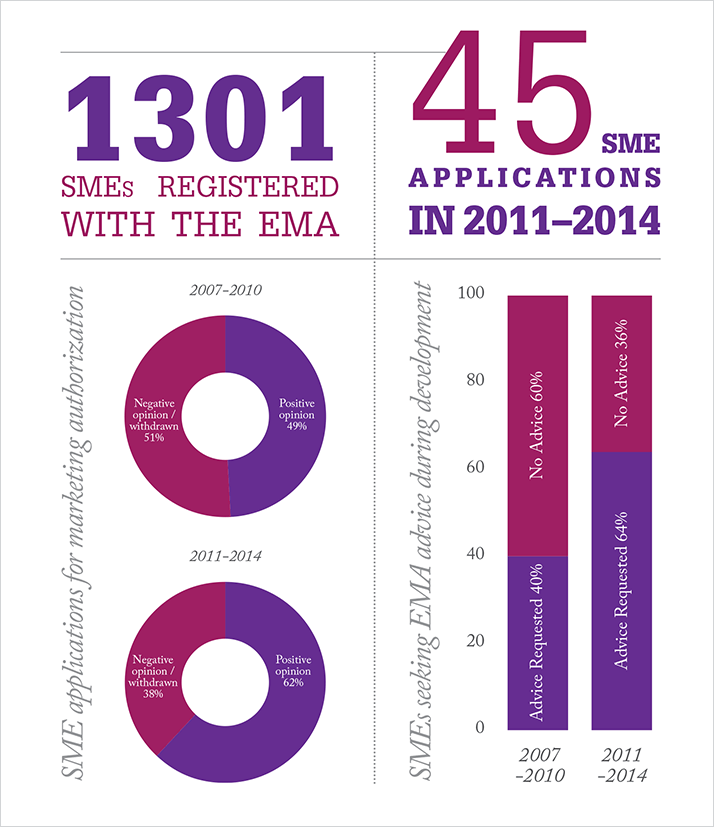
Increased uptake of the EMA’s advisory services, including scientific advice during development and biomarker qualification, seems to have led to increased success rates for small and medium-sized enterprises (SMEs), according to the EMA’s annual SME Report. The report notes, “Initiating dialogue early and repeating it at major milestones is important to decrease the quality and clinical failure rate at time of marketing authorization review.” Notably, there are still areas for improvement, with quality and clinical documentation attracting the most objections – particularly in the biologics area.