We received a record number of entries for The Medicine Maker 2018 Innovation Awards, spanning machine makers, service providers, software developers, formulation experts, and more! The message? Innovation is abundant in every corner of the drug development industry! The top 16 innovations of 2018 were featured in The Medicine Maker in December, but how do we decide the grand winner?
Between December 2018 and March 2019, we asked readers to vote on the innovation they thought was the most groundbreaking. After counting up over two thousand votes, we are delighted to reveal the grand winner of 2018:
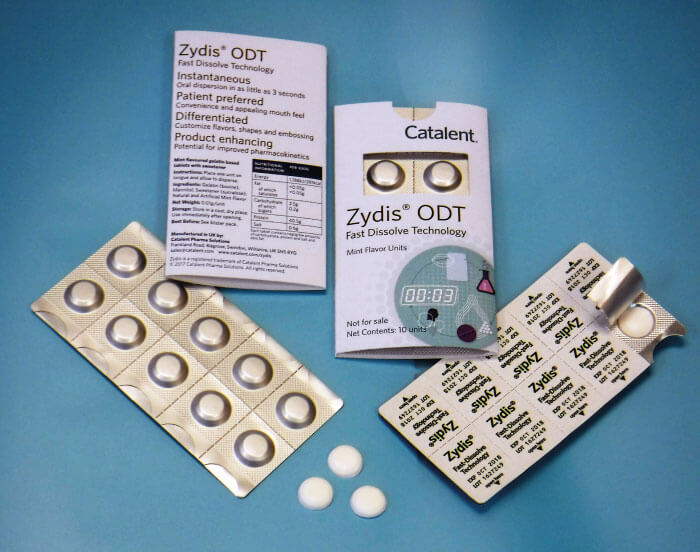
Zydis Ultra Coating Technology (Catalent Pharma Solutions)
Zydis Ultra Coating is a taste-masking technology that allows for increased drug load and taste masking to be incorporated into the company’s Zydis ODT dosage form. Zydis Ultra allows a patented and innovative drug coating to be introduced during a product’s formulation, enabling the dosage of active ingredient to be up to four times higher than a conventional Zydis ODT, while maintaining its speed of dispersion and superior mouth feel. More than 36 products have already been launched using Zydis.
The technology allows API particles to be coated within the Zydis ODT dosage form with a very thin (tens of microns) polymer. This coating is effective in taste masking the API without impacting oral disintegration time or its subsequent gastrointestinal dissolution performance. The coating technology uses acoustic mixing to bombard particles of the API with a micronized polymer; the energy produced increases the temperature of the API and polymer, allowing the polymer to form the coating over the API.
ODT technologies can help boost patient compliance – particularly in pediatric and geriatric populations where swallowing conventional tablets can be a problem. There are numerous products on the market that use ODT technology, but challenges in formulation exist where APIs have a bitter taste, or may burn and irritate the mouth. There are also limitations as to the API concentrations available within each dose. The increased drug loading possible with Zydis Ultra allows ODT platform technology to be expanded to many more compounds.
Second: syriQ BioPure
In second place was syriQ BioPure by Schott AG, Pharmaceutical Systems. These prefillable glass syringes have been designed specifically for the biologic market to keep sensitive drugs stable, ease administration, and shorten time to market.
Third: ÜBERcellFLEX
In third place was ÜBERcellFLEX, produced by G CON and IPS. This is a modular cleanroom space for cell therapy processing. Multiple units can be installed to scale up/out from phase 1 clinical production to commercial manufacturing and serve the needs of thousands of CAR-T patients per year.




