UK members of parliament recently expressed concerns that only around half of UK clinical trial results are being reported (1). And according to EU Trials Tracker, results for only 51.9 percent of those trials due in Europe have so far been reported (4072 out of 7846 trials) (2).
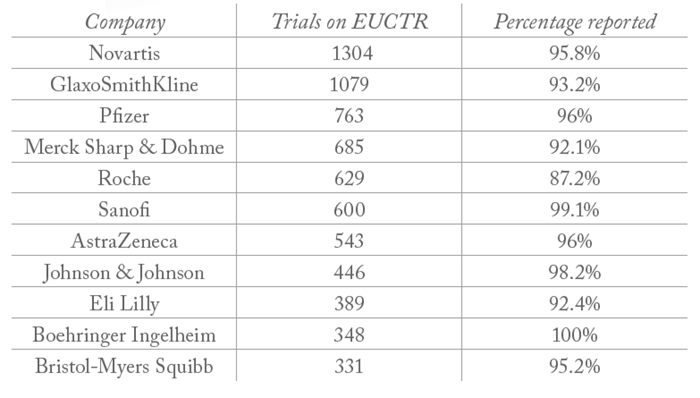
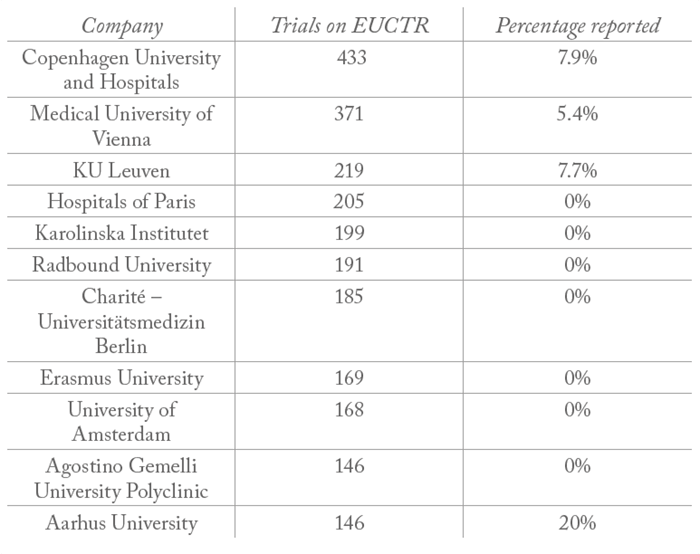
All clinical trials on the European Union clinical trials register (EUCTR) must report their results within a year of completion. And though very few companies report 100 percent of their trial results (meaning there is work to be done, see Table 1), big pharma is far from being the worst offender; hospitals and universities are significantly less likely to report their results – with many reporting no results at all (see Table 2).
References
- BBC News, “Unpublished medical research ‘a threat to public health’” (2018). Available at https://bbc.in/2Ror8I6. Accessed November 8, 2018. EU Trials Tracker. Available at https://eu.trialstracker.net. Accessed November 8, 2018.




