It is now widely accepted that biological sex can affect a person’s reaction to a drug. But historically, the standard clinical trial participant has been male. Is this still the case, or have things improved? Some recent studies and reviews report that barriers still remain, and that women continue to be underserved by clinical trials (1)(2). However, an analysis of FDA clinical trial registration data for frequently prescribed drug classes found that the inclusion of women increases from 22 percent in phase I trials to nearly 50 percent for phase II – III trials (3).
“The results of this investigation show that drug trials are appropriately designed regarding inclusion of men and women. Furthermore, the underrepresentation of women in trials as observed in the 1980s and before seems to be resolved for most drug trials that we investigated,” reports study co-author Robert Rissmann (4). But there is still some way to go. The authors acknowledge that the scope of their study is limited, and may not cover all the disease areas in which women may still be underrepresented. Other researchers have also reported that there is still room for improvement in our understanding of sex differences in response to some drugs (1) and some racial minorities continue to be underrepresented in various areas (3).
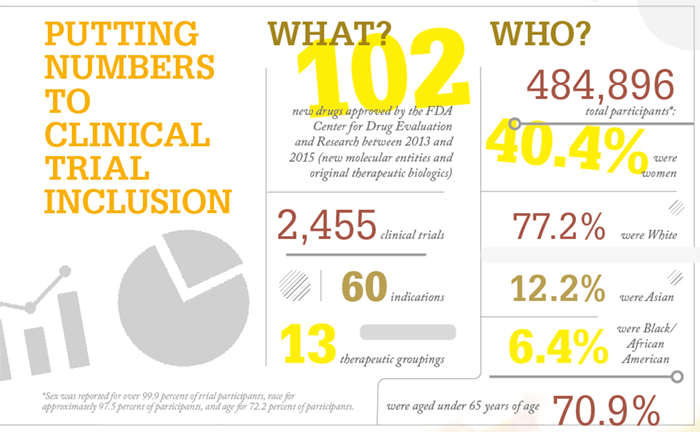
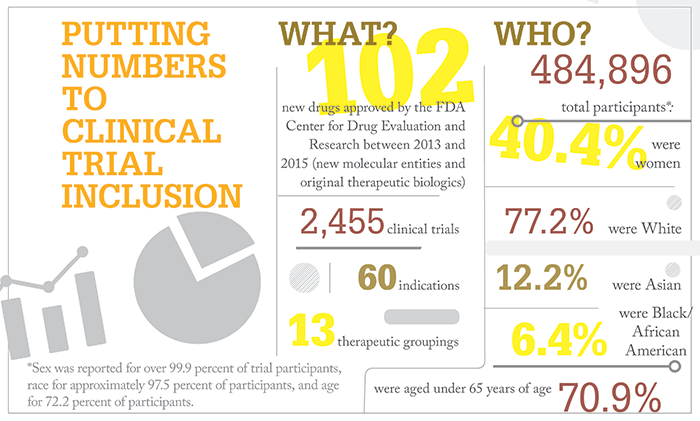
References
- KA Liu, NA Mager, “Women’s involvement in clinical trials: historical perspective and future implications”, Pharm Pract, 14, 708 (2016). PMID: 27011778. S Pal, “Inclusion of women in clinical trials of new drugs and devices”, US Pharm, 40, 21 (2015). A Chen et al., “Representation of women and minorities in clinical trials for new molecular entities and original therapeutic biologics approved by FDA CDER from 2013 to 2015”, J Womens Health, [Epub ahead of print] (2017). PMID: 29048983. EurekAlert!, “Are women really under-represented in clinical trials?”, (2017). Available at: http://bit.ly/wmntrials. Last accessed February 1, 2017.




