Data sharing can be a touchy subject, and there is a perception that pharma companies don’t exactly jump at the chance of disseminating clinical trial information. However, the European Federation of Pharmaceutical Industries and Associations (EFPIA) and Pharmaceutical Research and Manufacturers of America (PhRMA) recently published survey results showing that pharma companies are, in fact, open to data sharing when given the opportunity (1).
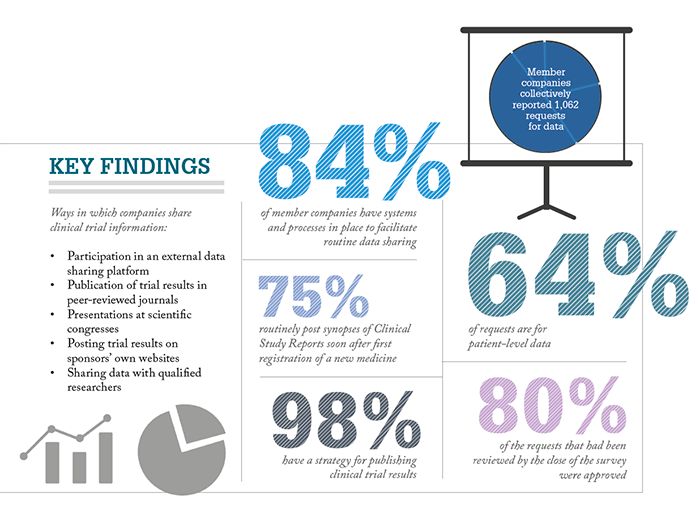
The two organizations aimed to boost the industry’s commitment to openness in 2013 when they released a set of principles outlining responsible data sharing, which covered five main areas: enhancing data sharing with researchers, enhancing public access to clinical study information, sharing results with patients who participate in clinical trials, certifying procedures for sharing clinical trial information, and reaffirming commitments to publish clinical trial results (2). The principles were offered as guidelines to be followed on a voluntary basis, but the survey results suggest that the principles have resulted in a positive effect – 98 percent of EFPIA and PhRMA member companies share more clinical data than is required by law or regulators. The 30-question survey was used to find out how much member companies had adhered to the principles, and to try and quantify their data sharing, between 2014 and 2016, with the overall aim of promoting the benefits and responsible use of information sharing.
References
- PhRMA, “EFPIA-PhRMA principles for responsible clinical trial data sharing”, (2017). Available at: http://onphr.ma/2AbRhWt. Last accessed December 6, 2017. EFPIA, “Principles for responsible clinical trial data sharing”, (2013). Available at: http://bit.ly/2jYAqeX. Last accessed December 6, 2017.




