Taking a promising drug all the way through to regulatory approval is a long, difficult process – and successes are rare. Most drugs fail before they reach the clinic, and most drugs that reach the clinic fail before approval. Diving deeper into the problem, the Biotechnology Innovation Organization (BIO) teamed up with Amplion (a biomarker business intelligence company) and BioMedtracker (a service that tracks a drug’s likelihood of approval by the FDA). BIO examined clinical trial success rates from 2006 to 2015, and 9,985 clinical and regulatory phase transitions were recorded and analyzed from 7,455 development programs, across 1,103 companies in the BioMedtracker database.
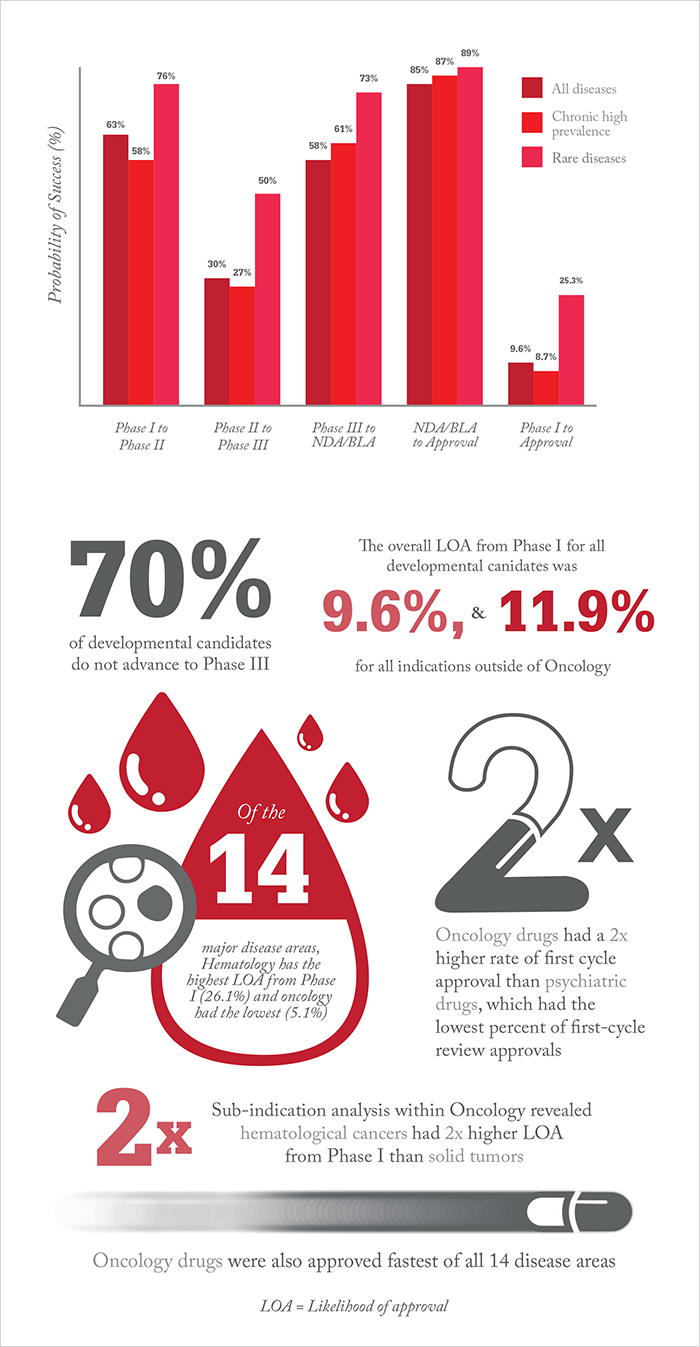
The study revealed that phase II clinical programs continue to experience the lowest success rate of the four development phases, with only 30.7 percent of developmental candidates advancing to phase III. For all diseases analyzed, only around 10 percent of drugs in phase I trials made it to approval. However, the study also showed that using biomarkers as selection criteria could dramatically increase success rates. Our infographic gives more information.
References
- D W Thomas et al., “Clinical development success rates 2006-2015”, (2016). Available at: http://bit.ly/1UfCn1S. Accessed June 9, 2016.




