In this second article of a short series Dr Paul Kippax is looking at the how recent innovations are substantially enhancing the value and informational productivity of some of our established and trusted analytical techniques. This time, the addition of multiple detectors to a GPC/SEC analysis…
The established technique: Gel permeation/size exclusion chromatography
For those working with materials with a chain-like molecular structure, such as proteins and polymers, gel permeation chromatography (GPC) or size exclusion chromatography (SEC) (alternative names for the same technique) is an essential tool. GPC/SEC is used to determine the molecular weight (MW) and molecular weight distribution of such samples, parameters that define performance. By altering MW, a polymer scientist can control material properties such as hardness, gas permeability and melting point; a food scientist can change the thickening or gelling behavior of a polysaccharide; and a biopharmaceutical formulator can determine the stability of a therapeutic protein.GPC/SEC is a two-step analysis. In the first step the dissolved sample is pumped through an appropriate chromatography column. This results in size fractionation on the basis of hydrodynamic size. Detection is the second step of the process and traditionally involves the measurement of sample concentration in each eluting fraction. The traditional GPC/SEC detector set-up therefore comprises a single concentration detector, either a refractive index (RI) or UV detector. Strictly speaking the set-up outlined above produces a hydrodynamic size distribution, raising the question of how to convert this into a far more valuable MW distribution. The conventional answer is to use a calibration standard. By running the column with a sample of known MW distribution, the elution time associated with a certain MW can be established and used to determine the MW distribution for an unknown sample. However, this approach will only be entirely accurate if the relationship between hydrodynamic size and MW is the same for the standard as for the sample – a fairly pronounced limitation.
The innovation: Adding detectors
Adding advanced detectors to a GPC/SEC set-up addresses the limitation posed by calibration, increases accuracy and boosts informational productivity. The addition of a light scattering detector is particularly helpful. Used in combination, a light scattering and a concentration detector allow the direct measurement of absolute MW, without any need for calibration. This means that accurate MW information can be gathered for any sample, even for new materials where there are no existing relevant standards. A viscometer can also be incorporated in the detector array. By generating intrinsic viscosity (IV) data, a viscometer also provides a measure of MW. However, if a light scattering detector is already in place, IV data can be used to elucidate changed structural characteristics such as branching. The case study below illustrates the value of direct MW measurement.The reveal: The same molecular weight or different?
Hypromellose (Hydroxypropyl Methylcellulose, HPMC) and its derivatives are used routinely in pharmaceutical formulation as, for example, controlled drug release agents. MW directly influences how these polymers perform in this application. MW distribution data for four samples of HPMC/HPMCAS (Hypromellose acetate succinate, a derivate) were measured by GPC/SEC. Results were generated using both a single detector set-up and a triple detector array. The conventional GPC/SEC results, from a single RI detector, indicate that all four samples have a similar molecular weight (see Table 1).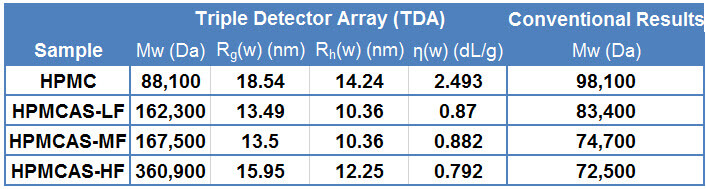
Table 1: Comparison of MW data reported using Triple Detection and conventional GPC/SEC. Conventional GPC/SEC reports only the molecular weight, however triple detection also provides a measure of molecular size (Rg(w) and Rh(w)) and intrinsic viscosity (ƞ(w)). However, the traces from the triple detector set-up (see Figure 1, data summarized in tabulated form) provide clear evidence that in fact these samples differ considerably in terms of their MW. Two of the derivatives have a weight averaged MW around double that of the HPMC while that of the third derivative is four times as high. Crucially there is no consistent correlation between the conventional results and those generated using the triple detector array.
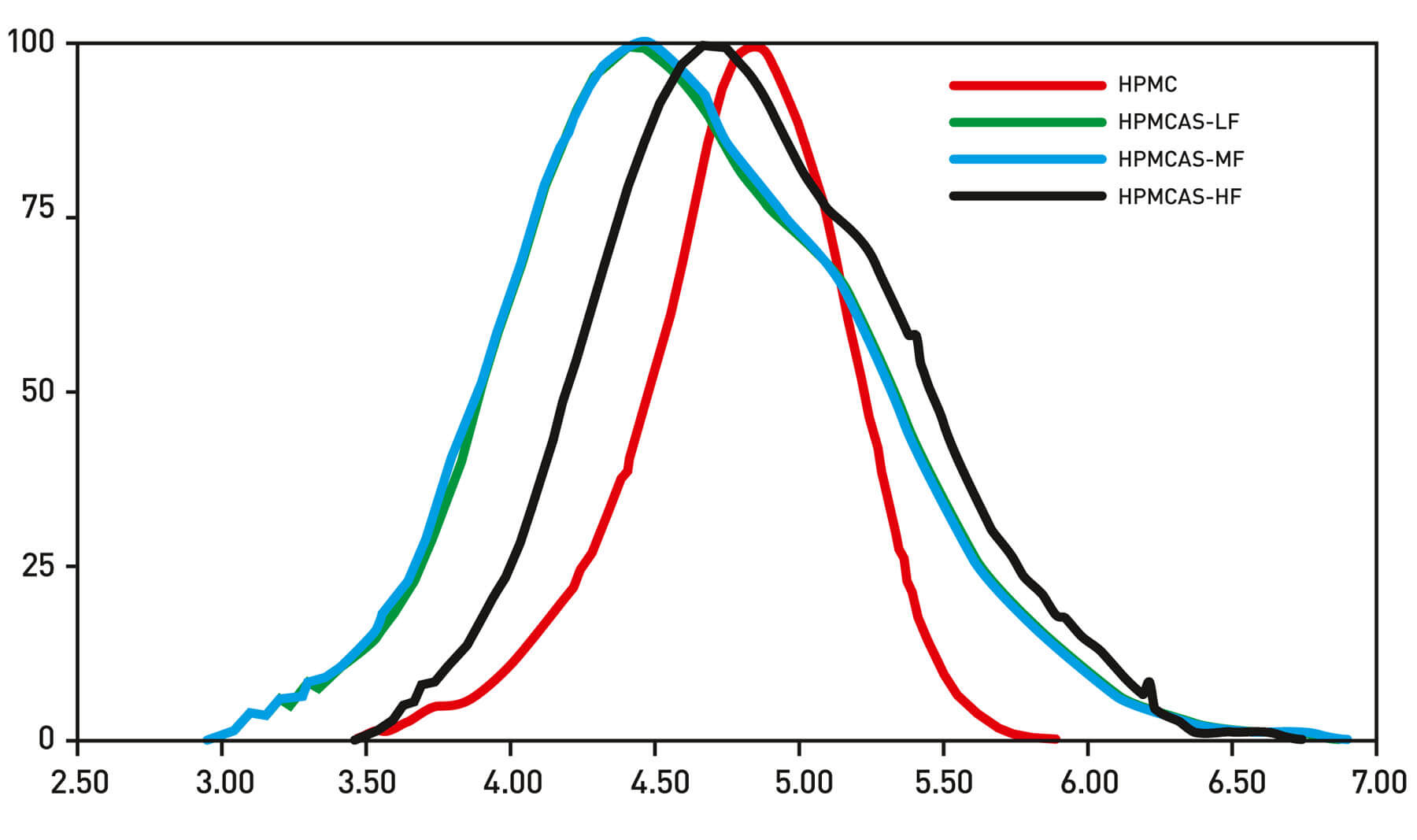
Figure 1: Triple detector GPC/SEC reliably and directly measures the absolute MW of polymeric excipients for controlled release, providing data that supports identification of a suitable candidate for any specific formulation. Here then, multiple detection GPC/SEC reveals the MW data that is critical for effective formulation and control of the clinical efficacy of a tablet, eliminating the inconsistent inaccuracies associated with using only a single detector.
Malvern Instruments provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations and our commitment to maximizing the potential of established techniques. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. Headquartered in Malvern, UK, Malvern Instruments has subsidiary organizations in all major European markets, North America, Mexico, China, Japan and Korea, a joint venture in India, a global distributor network and applications laboratories around the world. www.malvern.com severine.michel@malvern.com





