Introduction
Taylor Dispersion Analysis (TDA) is an orthogonal technique for sizing and stability studies of biomolecules in solution. Studies have shown that hydrodynamic radius (Rh) measurements from TDA on monodisperse systems such as monoclonal antibodies (mAbs), are comparable to data obtained using the well-established and complementary technique of Dynamic Light Scattering (DLS). Protein stability is one of the primary concerns during biopharmaceutical development, as it negatively impacts upon immunogenicity and potency of the biotherapeutic. Screening of candidate molecules for developability under a variety formulation conditions is therefore a critical aspect in early stages of the development pipeline.It is important to monitor any potentially adverse effects that buffer matrices, excipients and surfactants may have on the molecule of interest, such as changes in conformational stability and self-association. However observing changes occurring specifically to the target molecule can be analytically difficult, complicated by the complex background of the excipients themselves. Notably for protein stability assessment under formulation conditions, the use of TDA with a matched sample buffer effectively renders excipients and surfactants invisible, enabling label-free characterization of target biomolecules in complex solutions. This is in contrast to light scattering measurements for example, where data can become more and more dominated by scattering interference from excipients in the formulation. As an important biotherapeutic used in the treatment of Diabetes Mellitus, Insulin provides an interesting case study for the use of TDA as a technique for characterizing the hydrodynamic radius (Rh) and self-association and stability of protein-based biopharmaceuticals in complex solutions. The self-association of Insulin is fundamental to the protein’s function within the body; hexameric Insulin is the more stable form used to store the protein within cells, whereas monomeric Insulin is the active form that binds to Insulin receptors. Identifying the oligomeric state of Insulin in a specific formulation is therefore important in predicting the stability of the formulation. Hexamers are formed through the self-association of Insulin monomers into dimers, which combine to form hexamers. Changes in Insulin’s oligomeric state can be observed as a change in size, where smaller sizes correspond to lower order oligomers. Changes to environment can cause changes to the protein itself, and Insulin can be found in a number of different oligomeric states under different formulation conditions. In this study, Taylor Dispersion Analysis (TDA) has been used to assess the oligomeric state and stability of Insulin under varying buffer and excipient conditions. TDA is a microcapillary flow-based technique, whereby a nanoliter volume sample pulse is injected into a laminar flow of matched sample buffer. Analysis of the time-evolved concentration profile (or Taylorgram) from UV absorption allows the molecular diffusion coefficient and hence hydrodynamic radius of solute molecules to be determined.
Experimental
- Insulin (Sigma Aldrich, UK) was prepared in a number of different buffers, filtered (0.02µm, Fisher Scientific)
- Buffer preparations: PBS; PBS + 0.5M urea; PBS + 0.18% EDTA; 0.1M HCl + 10mM NaCl
- Buffer exchange to remove Zinc – EDTA was performed in Miditrap columns in accordance with the manufacturer’s instructions (GE Healthcare)
- TDA measurements were performed on a Malvern Viscosizer at 20ºC with a 214nm wavelength filter and an uncoated capillary (default measurement settings for Sizing were used throughout)
- DLS measurements were performed on a Malvern Zetasizer Nano at 20ºC
- Solution concentrations were determined by NanoDrop 2000 (Thermo Scientific)
Results and Discussion
Effect of formulation buffer on oligomeric state Figure 1 shows the variation in self-association and oligomeric state of Insulin in a selection of different formulation buffers as measured by TDA. These data follow the same trends that are well characterized within the literature.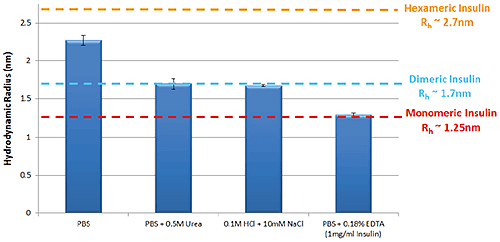
Figure 1: Hydrodynamic radius of Insulin measured by TDA in a range of buffer conditions. Insulin concentration is 2mg/ml (unless stated) Improving thermal stability of Insulin through the addition of Arginine Arginine is an excipient added to formulations in order to improve thermal stability6. Figure 2 is a thermal trend plot measured using DLS (Zetasizer Nano), showing how the addition of Arginine increases the temperature at which Insulin aggregates.
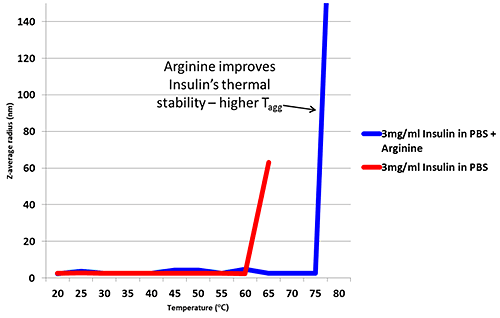
Figure 2: Thermal trend measured by DLS (Zetasizer Nano) showing an increased aggregation temperature for Insulin in PBS with the addition of Arginine DLS provides a useful tool for monitoring thermal stability, but when measuring changes to a molecule of interest, light scattering results can be influenced by excipients particularly when the excipient is of similar size to the target biomolecule. Figure 3 shows how the Arginine present in the buffer is picked up as a separate intensity peak in DLS, whereas the TDA Taylorgram is not affected by the addition of excipients. TDA is able to selectively monitor the molecule of interest, independent of formulation components. Note the Malvern Viscosizer uses twin window detection methodology for TDA, hence the two peaks shown in the Taylorgrams in Figure 3 are two measurements from the same sample pulse that are temporally-separated at different detection points along the microcapillary.
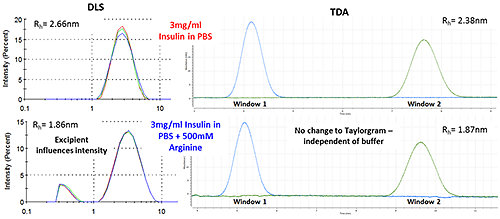
Figure 3: DLS (Intensity vs size plots) and TDA Taylorgrams (UV Absorbance vs time plots) for solutions of 3mg/ml Insulin in PBS with and without the addition of Arginine.
Conclusion
The influence of formulation components such as excipients and surfactants on protein stability and self-association in solution is important to understand, as biotherapeutic products generally require complex formulations.
Light scattering techniques are well established as a screen for early stage developability, but the influence of complex mixtures of excipients and surfactants in the formulation matrix can interfere significantly with measurement data, from reducing measurement sensitivity (particularly at low protein concentrations) to masking the response from the target protein molecule.
Using Insulin as a case study, this Application Note demonstrates how Taylor Dispersion Analysis can be used to monitor self-association and oligomeric state for a variety of buffer conditions.
Notably, the use of specific UV wavelength detection and matched sample buffer effectively tunes TDA to be selective for the target protein molecule, and renders the formulation components invisible in measurement data.
TDA is particularly well suited as an orthogonal technique for early stage developability screening for biotherapeutics, especially as this automated technique is applicable at low concentration with ultra-low sample volume requirements.
Malvern Instruments provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems. These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries. Used at all stages of research, development and manufacturing, Malvern’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency. Our products reflect Malvern’s drive to exploit the latest technological innovations and our commitment to maximizing the potential of established techniques. They are used by both industry and academia, in sectors ranging from pharmaceuticals and biopharmaceuticals to bulk chemicals, cement, plastics and polymers, energy and the environment. Malvern systems are used to measure particle size, particle shape, zeta potential, protein charge, molecular weight, mass, size and conformation, rheological properties and for chemical identification, advancing the understanding of dispersed systems across many different industries and applications. Headquartered in Malvern, UK, Malvern Instruments has subsidiary organizations in all major European markets, North America, Mexico, China, Japan and Korea, a joint venture in India, a global distributor network and applications laboratories around the world. www.malvern.com severine.michel@malvern.com

References
- A. Lavoisier, J-M Schlaeppi, ‘Early developability screen of therapeutic antibody candidates using Taylor Dispersion Analysis and UV area imaging detection’ mAbs 7:1, 77-83 (2015)
- A. Hawe, W.L. Hulse, W. Jiskoot, R.T. Forbes, ‘Taylor Dispersion Analysis compared to Dynamic Light Scattering for the size analysis of therapeutic peptides and proteins and their aggregates’ Pharm Res 28: 2302-2310 (2011)
- A.M. Gualandi-Signorini, G. Giorgi, ‘Insulin formulations – a review’ European Review for Medical and Pharmacological Sciences 5: 73-83 (2001)
- Malvern White Paper ‘Understanding Taylor Dispersion Analysis’ www.malvern.com
- A. Ahmad, I.S. Millett, S. Doniach, V.N. Uversky, A.L. Fink, ‘Stimulation of Insulin Fibrillation by Urea-induced Intermediates’ Journal of Biological Chemistry 279: 14999-15013 (2004)
- M.M. Varughese, J. Newman, ‘Inhibitory Effects of Arginine on the Aggregation of Bovine Insulin’ Journal of Biophysics Volume 2012, Article ID 434289, 7 pages (2012)





